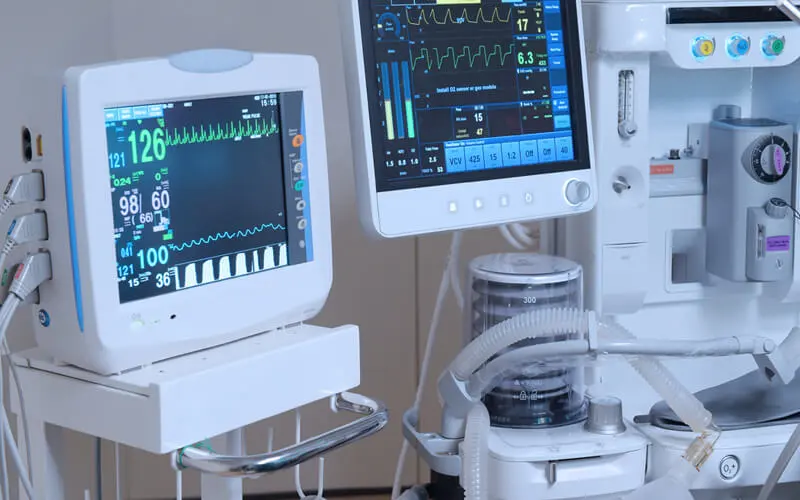
Medical devices encompass a broad category of instruments, machines, implants, and other tools used in healthcare settings for the diagnosis, treatment, monitoring, and management of medical conditions. These devices play a crucial role in modern medicine, enabling healthcare professionals to deliver accurate diagnoses, perform intricate surgical procedures, monitor patient health parameters, and improve overall quality of care. The field of medical devices intersects with various disciplines, including electronics, engineering, medicine, and biology, to develop innovative solutions that address the diverse needs of patients and healthcare providers.
Medical devices range from simple instruments like thermometers and stethoscopes to complex systems such as MRI machines and implantable pacemakers. They can be categorized based on their intended use, complexity, and level of invasiveness. Medical devices often incorporate advanced electronics, sensors, microprocessors, and software to perform their functions accurately and efficiently. These devices undergo rigorous testing and regulatory scrutiny to ensure safety, efficacy, and compliance with industry standards and regulations.
Applications
- Diagnostic Devices: Medical diagnostic devices are used to detect, measure, and monitor various health parameters, including blood pressure, glucose levels, cholesterol levels, and cardiac activity. Examples include blood glucose meters, electrocardiographs (ECG), ultrasound scanners, and X-ray machines.
- Therapeutic Devices: Therapeutic medical devices are designed to deliver treatments, therapies, and interventions to patients to alleviate symptoms, manage conditions, and promote healing. Examples include insulin pumps, nebulizers, dialysis machines, and ventilators.
- Surgical Instruments: Surgical medical devices are used by surgeons and healthcare professionals during surgical procedures to perform precise incisions, manipulations, and interventions. Examples include scalpels, forceps, surgical lasers, and robotic surgical systems.
- Implantable Devices: Implantable medical devices are surgically placed inside the body to provide therapeutic benefits, monitor physiological parameters, or replace dysfunctional organs or tissues. Examples include pacemakers, defibrillators, cochlear implants, and artificial joints.
- Monitoring Devices: Medical monitoring devices continuously track and record vital signs, physiological parameters, and patient activity to monitor health status, detect abnormalities, and provide timely interventions. Examples include cardiac monitors, pulse oximeters, fetal monitors, and sleep apnea monitors.
- Rehabilitative Devices: Rehabilitative medical devices assist patients in regaining mobility, strength, and function following injury, surgery, or illness. Examples include prosthetic limbs, orthotic braces, wheelchairs, and physical therapy equipment.
Industries Served
- Hospitals and Clinics: Medical devices are used in hospitals, clinics, and healthcare facilities for patient diagnosis, treatment, and monitoring across various medical specialties, including cardiology, neurology, orthopedics, and oncology.
- Home Healthcare: Many medical devices are designed for home use, allowing patients to monitor their health conditions, manage chronic diseases, and administer treatments without the need for frequent hospital visits.
- Ambulatory Care: Medical devices are utilized in ambulatory care settings such as ambulatory surgery centers, urgent care centers, and outpatient clinics to provide diagnostic tests, treatments, and minor surgical procedures.
- Long-Term Care Facilities: Medical devices are used in long-term care facilities, nursing homes, and rehabilitation centers to support the needs of elderly and disabled patients, including mobility aids, monitoring devices, and assistive technologies.
- Research and Development: The medical device industry also encompasses research and development organizations, academic institutions, and medical technology companies that innovate, design, and develop new medical devices and technologies to advance healthcare.
Target Areas and Considerations
- Regulatory Compliance: Medical devices are subject to stringent regulations and standards to ensure safety, efficacy, and quality. Regulatory bodies such as the U.S. Food and Drug Administration (FDA) in the United States and the European Medicines Agency (EMA) in Europe oversee the approval and marketing of medical devices.
- Clinical Validation: Medical devices undergo clinical trials and studies to demonstrate their effectiveness, safety, and performance before they are approved for use in clinical settings. Clinical validation involves rigorous testing, data analysis, and evaluation of patient outcomes.
- User Experience: Design considerations such as usability, ergonomics, and user interface play a crucial role in the adoption and acceptance of medical devices by healthcare professionals and patients. User-friendly devices improve efficiency, reduce errors, and enhance patient satisfaction.
- Interoperability: In the era of digital healthcare, interoperability is essential for seamless integration and communication between medical devices, electronic health records (EHR) systems, and healthcare IT infrastructure. Standardized data formats, protocols, and interfaces facilitate interoperability and data exchange.
- Data Security and Privacy: Medical devices collect and transmit sensitive patient health information, making data security and privacy paramount concerns. Robust cybersecurity measures, encryption techniques, and access controls safeguard patient data from unauthorized access, breaches, and cyber threats.
Medical devices play a critical role in modern healthcare by enabling early diagnosis, effective treatment, and improved patient outcomes. As technology continues to advance, the medical device industry will witness further innovation, integration of artificial intelligence, miniaturization of devices, and personalized medicine approaches to address evolving healthcare needs and challenges. Collaboration among healthcare professionals, industry stakeholders, regulators, and innovators is essential to drive the development, adoption, and utilization of safe, effective, and transformative medical devices that benefit patients worldwide.